People:
Joseph Pawlik
Graduate students
Information for undergrads and
prospective grad students
Science:
Marine chemical ecology
Giant barrel sponge Xestospongia muta
Marine invertebrate larval
biology
Photographic guide to sponges of the Caribbean
Courses:
BIO 318:
Invertebrate
Zoology
BIO 501: Science as a Profession
| HOME |

Click HERE to watch Dr. Pawlik give a 17 minute talk about his research (from a symposium at the Smithsonian Institution, Washington DC, 24 May 2010).
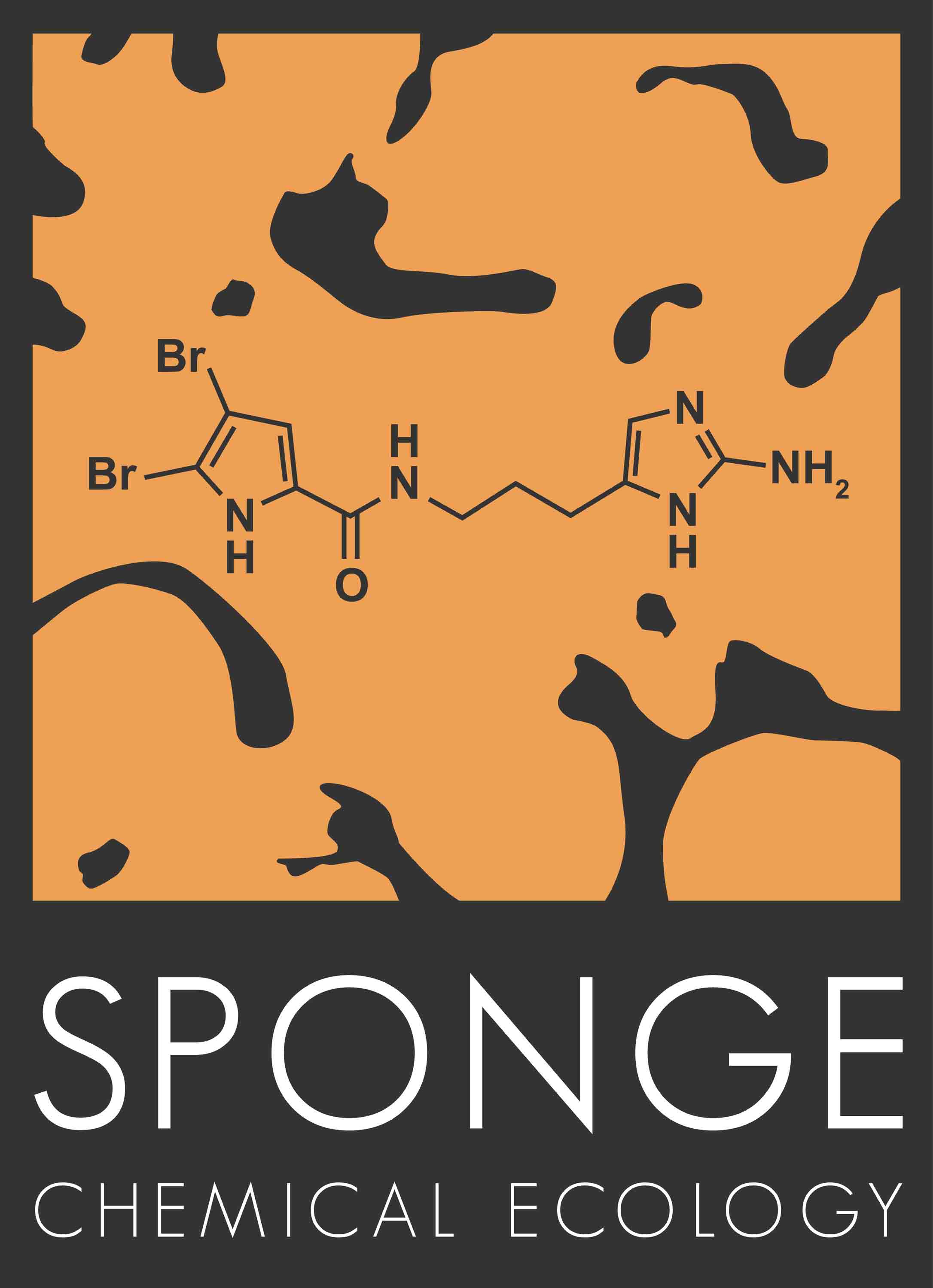
Assessing the Chemical Defenses of Caribbean Sponges
Before the chemical ecology of the system was
better understood, it was believed that consumers had little effect on Caribbean
sponge communities (Randall & Hartman 1968). Randall and Hartman concluded
their influential study with: “No strong evidence is
provided by our data that fish predation is a significant factor in limiting
sponge distribution in the West Indian region.”
We began this research program in the mid-1990s by surveying the chemical antipredatory defenses of 73 species of Caribbean sponges using a common generalist fish (bluehead wrasse) as an assay organism (Pawlik, et al. 1995). The majority (69%) of sponge species yielded deterrent extracts, but, to our surprise, there was considerable interspecific variability in chemical defenses. Further, we found little evidence of a defensive role for glass spicules or organic skeleton components (Chanas & Pawlik 1995, 1996, Jones et al. 2005). Reef sponges generally yielded more deterrent extracts than sponges from mangrove or grassbed habitats, but 4 of the 10 most common sponges on reefs yielded palatable extracts. Most importantly, we discovered that extracts of sponge species that were palatable to generalists were the same species eaten by sponge-eating fishes (Pawlik 1997,1998). Besides sponges, we investigated defensive strategies in ascidians (Pisut & Pawlik 2002, Odate & Pawlik 2007) and gorgonian corals (O’Neal & Pawlik 2002, Epifanio et al. 2007), finding no clear defensive trade-off between organic acids and secondary metabolites in the former, and little evidence of structural defenses in the latter (both of these were undergraduate research projects). We have also investigated the chemical defenses of sponges against less-common invertebrate predators (Waddell & Pawlik 2000a,b), and found patterns similar to those we had previously described for fish predators.
Isolating and identifying chemical defenses against fish predators.
Focusing our attention on sponge species with strongly deterrent extracts,
we used bioassay-guided isolation techniques to identify chemical defenses of
several species, including pyridinium salts in Amphimedon spp. (amphitoxin,
Albrizio et al. 1995), brominated alkaloids in Agelas and Axinella
spp. (oroidin, Chanas et al. 1996; stevensine, Wilson et al. 1999; Lindel et al.
2000; Assmann et al. 2000), terpenoid glycosides in Erylus and
Ectyoplasia spp. (Kubanek et al. 2001, 2002), brominated tyrosine
derivatives in Aplysina spp. (fistularin, Puyana et al. 2003), sulfated
phenolic terpenoids in Siphonodictyon coralliphagum (Grube et al. 2007)
and sulfated sterols in Phorbas amaranthus (amaroxocanes, Masuno et al.
2004, Morinaka et al. 2009). Collaborations with synthetic chemists allowed us
to test structure-activity relationships of the pyrrole-imidazole alkaloids that
defend Agelas spp. (Lindel et al. 2000). Testing a long-standing
assumption, we found that the “putrid stench” volatile metabolites from sponges
of the genus Ircinia were not the basis for their potent chemical
defense, but rather a group of furanosesterterpene tetronic acids (Pawlik et al.
2002). These studies went far beyond our initial aquarium-based survey
experiments, employing field assays with natural populations of reef fishes, and
using geographically distant sponge samples to investigate intraspecific
variation in defenses.

Chemical defenses against competitors and pathogens. The "defensive" part of the term "chemical defense" is often used to refer solely to predator-deterrence, but there are alternative defensive roles of invertebrate secondary metabolites, including antifouling and anti-overgrowth functions. We developed new techniques for testing these roles using gel materials as media for the containment and release of crude extracts and purified compounds. Metabolites were incorporated into gels at natural volumetric concentrations that are slowly released into seawater, thereby providing a more ecologically realistic field assay technique (Henrikson & Pawlik 1995, Kubanek et al. 2002, Pawlik et al. 2007). Adapting this gel technique, we have developed assay methods for assessing the allelopathic activity of sponge metabolites against other sponges and ascidians (Engel & Pawlik 2000), and against the colonizing stages of a range of environmental and pathogenic marine bacteria (Newbold et al. 1999, Kelly et al. 2003, 2005).
Transforming our understanding of Caribbean reef ecology. Prior to our investigations, the conventional view of sponge ecology on Caribbean reefs was that there was no top-down control of sponge populations, because only a few fish species spread their predatory activities over a wide variety of sponge species (Randall & Hartman 1968). Our results demonstrated that sponge-eating fishes are not “smorgasbord” feeders, but preferentially select sponge species that lack deterrent chemistry (Pawlik 1997, 1998). Only a few sponge species make up most of the diet of four of the six major spongivores, and these sponge species are notable because their crude extracts are palatable to generalists, and they are among the ten most common sponge species on Caribbean reefs (Pawlik et al. 1995; Pawlik 1997). We also discovered that sponge-eating fishes prefer to eat a suite of sponges that are grazed completely from the open reef; as a result, these sponges are found only in refuge habitats, such as in cryptic locations on the reef, or in mangrove environments (Dunlap & Pawlik 1996,1998). When mangrove sponges were transplanted to the reef alongside similarly transplanted reef sponges, spongivores quickly located mangrove species and consumed them, leaving reef sponges untouched (Dunlap & Pawlik 1996; Pawlik 1998). To our surprise, parrotfishes took 34.8% of the bites on the mangrove sponges. We subsequently discovered that preferred mangrove species could also be found under reef rubble, and when rubble was turned-over, these species were quickly eaten, as they had been in the transplant experiments (Dunlap & Pawlik 1996). Tissue from these preferred species made up 8-14% of the guts of 4 of the 6 most common spongivores surveyed by Randall & Hartman (1968). Despite our inability to find these cryptic sponges growing in apparent locations on the reef, spongivores are locating enough of these species, either as they grow out of refugia or as rubble is disrupted, that they constitute ~10% of spongivore diets (Pawlik 1997, 1998). We proposed, and have since further developed, a conceptual, top-down ecosystem model for sponges on Caribbean coral reefs based on assay data from over 80 species (Pawlik et al. 1995, Loh & Pawlik, 2010) that separates sponges into three categories: preferred species that are grazed completely from the reef and restricted to refugia inside the reef framework (poor tolerance and defense), palatable species that are common on the reef and tolerate grazing (well-developed tolerance, poor defense), and defended species that are protected from predation by chemical defenses (poor tolerance, well-developed defense; Pawlik 1997, 1998; Pawlik et al. 2008). We have established that there is a clear hierarchy of competition among reef sponges (Engel & Pawlik 2005a), developed novel assay systems for identifying the chemical basis of allelopathy between sponge species (Engel & Pawlik 2000; Kubanek et al. 2002) and have begun investigating allelopathic interactions between sponges and corals (Pawlik et al. 2007, Part 1C below). Based on resource availability theory from plant-herbivore ecology that predicts slow-growing plants should invest heavily in defenses while fast-growing plants will instead tolerate and circumvent herbivory through growth or reproduction, we proposed that palatable reef sponges should allocate resources otherwise used for the synthesis and storage of chemical defenses to grow, heal wounds, or reproduce faster than sponge-eating fish can consume them. These predictions proved remarkably accurate, supporting the dominance of top-down control in the evolution of Caribbean sponges: palatable species healed faster than defended species (Walters & Pawlik 2005), grew faster (Leong & Pawlik 2010b), and in some cases produced more propagules (Lindquist et al. 1997, Leong & Pawlik, in prep), although the interactive complexity of these trade-offs make multispecies comparisons of single factors difficult (Pawlik et al. 2008).
Click HERE for references. Funding provided by...


